Published on Aug 18, 2015
IASbaba's Daily Current Affairs
IASbaba's Daily Current Affairs - 18th August, 2015
Archives
IASbaba's Daily Current Affairs- 18th August, 2015
INTERNATIONAL
India’s ties with West Asia : An analysis (Part I)
- India’s relationship with the Middle East or the official United Nations term of
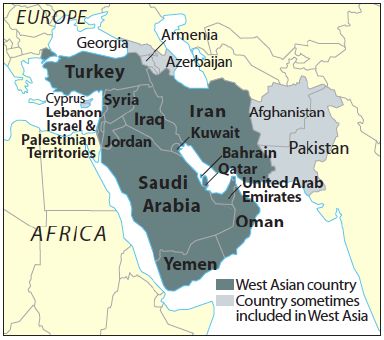 "West Asia" is driven by the cold, hard logic of realism.
"West Asia" is driven by the cold, hard logic of realism. - India’s foreign policy establishment is devoting no small amount of effort to understand their country's role in a politically unstable, but exceedingly important, area of the world for Indian interests.
- The government is poised to launch its high-level engagements with West Asia, which is in the intense of changing political dynamics in the wake of growing threat from Islamic State (IS) and Iran nuclear deal.
- Also the government is looking at leveraging the position India enjoys as a country with world’s second largest Muslim population and which has robust ties with key players in the region, including UAE, Saudi Arabia, Israel and Iran, to play a befitting role.
India and UAE economic relations: An analysis
- It is estimated that about 2.6 million Indians live in UAE with a remittance value of about $10 billion.
- UAE, is India's top trading partner in the entire West Asia North Africa (WANA) region, as it alone represents 25% of India's export to Gulf Cooperation Council(GCC) Indian exports to the UAE account for 5% of India's global exports.
- The main items of exports from India to UAE includes mineral fuels, natural or cultured pearls, cereals, gems and jewellery, manmade yarn, fabrics, metals, cotton yarn, marine products, machinery and equipment, plastic and linoleum products, tea and meat .
- In 2008-09, India emerged as one of the biggest trade partner of the UAE with bilateral trade between the two countries exceeding US$1.5 billion.
- India imports about 10% of its crude oil demand from UAE.
- Major items of imports from UAE include mineral fuels, mineral oils, natural or cultured pearls, precious or semi-precious stones, metal ores & metal scrap, sulphur and unroasted iron pyrites, electrical machinery and equipment and parts thereof, iron and steel etc.
Way ahead in India UAE economic relations : (Iasbaba’s view)
- There is a potential of about $1 trillion investments into India from UAE.
- Infrastructure development and real estate offer tremendous opportunities for UAE businesses in India.
- In agriculture sector, India can benefit from cold storage and warehousing network technology from UAE.
- Tourism sector is one of the areas that has good potential for future growth, especially medical tourism. Immigrants coming to India are already utilising Indian health services, including the ayurvedic establishments and spas.
- Another area with considerable scope for cooperation in tourism is construction and maintenance of hotels. There is good scope for UAE to invest in the over-all tourism sector in India, which would help pull tourists visiting the UAE to India as well.
- Since UAE is focusing on knowledge based industries and with India emerging as world leader in space, agriculture, pharmaceuticals and bio-technology, there is considerable scope for cooperation in technology transfer, R&D and for joint ventures.
Background :
- Gulf Cooperation Council: It is a regional intergovernmental political and economic union consisting of all Arab states of the Persian Gulf. Its member states are Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates.
Connecting the dots:
- Eventhough UAE’s economic, political and cultural links with India, dates back to more than a century , the relationship has not matured to the extent that was expected. Critically analyse.
ECONOMICS
Diminishing credibility of Indian Pharma
- A foreign drug safety regulator identifies serious data integrity issues provided by an Indian company.
- Yet, instead of investigating the accusations in a transparent manner, the Centre predictably cries foul.
- Instead of crying foul, Indian regulators should be examining the evidence and acting upon it.
On What basis did EU ban Indian drugs?
- The European Commission stresses that the decision concerning a ban on 700 generic drugs was based on scientific and not trade considerations and in accordance with the advice of the scientific committee of the European Medicines Agency (EMA).
- The EU-wide step came following several country-specific bans, in Germany and France among others.
- Indian industry has argued that these medical products have been in use in Europe for several years without any specific vigilance reports against them - though the Europeans may well retort that a vitiated testing and approval process is sufficient reason for a ban, even in the absence of compound-specific reports.
- India has been in touch with European drug regulators to allay their apprehensions about the testing procedures in the Hyderabad facility.
How does this move effects Indian pharmaceutical industry?
- The country could lose about $1-1.2 billion worth of drug exports because of the decision taken by the European Commission to ban the drugs, according to Pharmaceuticals Export Promotion Council (Pharmexcil).
- India exported $15.4 billion worth of pharmaceutical products in 2014-15, with Europe accounting for $3 billion, or 20 per cent of the total.
- Out of the $3 billion, exports of generic medicines constituted about $1 billion and drug ingredients accounted for the rest.
Is the “quick fix’’ culture and “chaltahai’’ attitude of India Increasing?
- If Indian companies have to export to western markets, where the returns are much higher than African or Latin American countries, they will have to meet their stringent standards.
- There is no question of any government compromising on quality in the case of a product as critical as medicine.
- The Indian regulators will also have to pull up their socks and ensure that the best global standards are being met instead of waiting for their western counterparts to point out the mistakes and then go on the defensive.
- The "non-tariff barriers’’ argument that is often raised to defend Indian goods against western curbs is not easy to accept in relation to faults found in the production of life-saving drugs .
- A few black sheep can give the entire nation a bad name and a hawk’s eye needs to be kept to check such malpractices.
- The US government has also been mounting pressure on India to tighten patent laws. However, the fact that MNCs have been “ever-greening’’ patents with cosmetic changes in their drugs merely to keep out competition has strengthened India’s case.
- Several court rulings have gone against the MNCs on the issue. India cannot afford to fritter away the competitive advantage that it has painstakingly built up in the pharma sector over the years and the huge benefit that flows from it to the sick and suffering.
Is Indian Pharma and drugs safety being recognized as the symbol of LOBBY & MANIPULATION?
- The fact that another Indian clinical research organization based in Chennai landed in trouble this time with the World Health Organization (WHO) makes matters even worse.
- The “notice of concern’’ issued by the United Nations health agency last month comes close on the heels of the 700 generic drugs being withdrawn in Europe.
- WHO stated that there had been critical lapses in a trial of HIV drugs, including the fact that two-thirds of patients' electrocardiograms (ECGs) turned out to be fake as details and dates had been changed by the Chennai firm.
- The WHO inspectors also criticised the standard of record-keeping in the trial, including apparent attempts to conceal documents from inspectors.
- Multinational pharma companies, that sell branded drugs at exorbitant prices, represent a formidable lobby opposed to Indian competitors who they have often accused of infringing patent rights.
How the Drug Circulation in the world does actually works?
- A country’s drug regulator is well within its rights to decide which medicines are suitable for its population and which ones aren’t.
- What matters is whether it has systems in place to discharge its responsibilities.
- In what pharmaceutical companies call “established” markets such as western Europe and the US, where new drugs are almost always launched first, regulatory agencies approve a drug based on the results of safety and efficacy trials on human volunteers.
- As no drug is entirely free of side-effects, agencies take a decision based on the risk-benefit ratio.
- It is the regulator who decides that certain risks are acceptable when compared with the drug’s benefit.
How far is the DRUG REGULATOR responsible?
- Regulators in so called emerging markets such as India take their cues from the West.
- They usually prescribe a safety and efficacy trial on roughly 100 Indian volunteers so long as the drug is approved in its home country or a major market.
- Since India is predominantly an off-patent drugs market, a number of companies other than the drug’s innovator have traditionally made their copies of the drug available here, sometimes before the innovator.
- Since the regulatory barriers to entry are relatively low, India has hundreds of companies selling thousands of drug formulations.
What are the Major Pitfalls in the Indian Approach?
- First, while in most cases drugs pass muster with all major regulators, in some cases that does not happen.
- So one country may approve a new drug, another might not for any number of reasons, including politics.
- Second, more relevant to the issue of banned drugs is that a medicine may prove unsafe after it has been marketed.
- Even a drug approved after rigorous clinical trials might throw up unacceptable risks when given to a larger population.
- That is why well-regulated markets have systems to monitor new drugs post-approval.
- This is usually a combination of post-market surveillance of new drugs and regulatory activity to get information about side-effects from doctors, hospitals, patients etc.
- The system works imperfectly, but it does work.
Connecting the Dots:
- Why India still sells medicines banned elsewhere in the world?
- If two major market regulators (USA & France) take contradictory positions on ban of drugs, what should India do? (Example drug pioglitazone)
